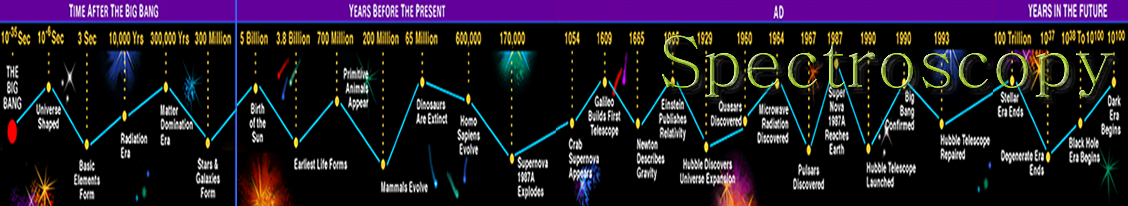
Infrared spectroscopy (IR Spectroscopy) is the subset of spectroscopy that deals with the Infrared part of the electromagnetic spectrum. Radiation in this region can be utilized in organic structure determination by making use of the fact that it is absorbed by interatomic bonds in organic compounds. Chemical bonds in different environments will absorb varying intensities and at varying frequencies. As with all spectroscopic techniques , it can be used to identify a compound and to investigate the composition of a sample. Thus IR spectroscopy involves collecting absorption information and analysing it in the form of a spectrum -- The frequencies at which there are absorptions of IR radiation ("peaks" or "signals") can be correlated directly to bonds within the compound in question. Infrared spectroscopy correlation tables are also tabulated in the literature. An invaluable tool in organic structure determination and verification involves the class of electromagnetic (EM) radiation with frequencies between 4000 and 400 cm -1 (wavenumbers).
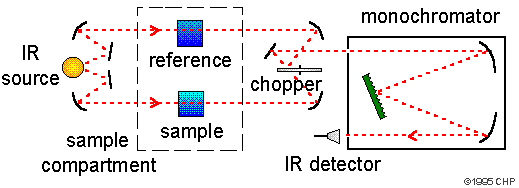
Nuclear magnetic resonance ( NMR ) is a physical phenomenon based upon the magnetic properties of an atom's nucleus . All nuclei that contain odd numbers of nucleons and some that contain even numbers of nucleons have an intrinsic magnetic moment . The nuclei of many elemental isotopes have a characteristic spin ( I ). Some nuclei have integral spins (e.g. I = 1, 2, 3 ....), some have fractional spins (e.g. I = 1/2, 3/2, 5/2 ....), and a few have no spin, I = 0 (e.g. 12 C, 16 O, 32 S, ....). Isotopes of particular interest and use to organic chemists are 1 H, 13 C, 19 F and 31 P, all of which have I = 1/2. Since the analysis of this spin state is fairly straightforeward, our discussion of nmr will be limited to these and other I = 1/2 nuclei. The most commonly used nuclei are hydrogen-1 and carbon-13, although certain isotopes of many other elements nuclei can also be observed. NMR studies a magnetic nucleus, like that of a hydrogen atom ( protium being the most receptive isotope at natural abundance) by aligning it with a very powerful external magnetic field and perturbing this alignment using an electromagnetic field . Tables are used to show proton resonance. Home
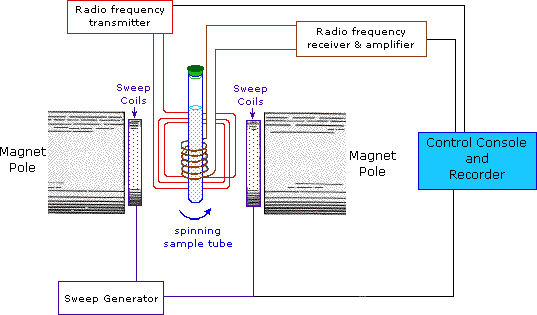
A mass spectrometer is a device that measures the mass-to-charge ratio of ions. This is achieved by ionizing the sample and separating ions of differing masses and recording their relative abundance by measuring intensities of ion flux. A typical mass spectrometer comprises three parts: an ion source, a mass analyser, and a detector system. Atoms can be deflected by magnetic fields - provided the atom is first turned into an ion. Electrically charged particles are affected by a magnetic field although electrically neutral ones aren't. The sequence is : Stage 1: Ionisation The atom is ionised by knocking one or more electrons off to give a positive ion. This is true even for things which you would normally expect to form negative ions (chlorine, for example) or never form ions at all (argon, for example). Mass spectrometers always work with positive ions. Stage 2: Acceleration The ions are accelerated so that they all have the same kinetic energy. Stage 3: Deflection The ions are then deflected by a magnetic field according to their masses. The lighter they are, the more they are deflected. The amount of deflection also depends on the number of positive charges on the ion - in other words, on how many electrons were knocked off in the first stage. The more the ion is charged, the more it gets deflected.Stage 4: Detection The beam of ions passing through the machine is detected electrically.
Fragmentation patterns are used to explain the ions which are seen in the spectra. Home
http://www.chemguide.co.uk/analysis/masspec/howitworks.html#top
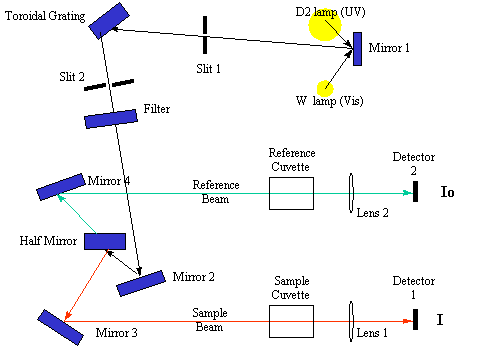
Ultraviolet-visible spectroscopy or ultraviolet-visible spectrophotometry ( UV/ VIS ) involves the spectroscopy of photons and spectrophotometry . It uses light in the visible and adjacent near ultraviolet (UV) and near infrared (NIR) ranges. In this region of energy space molecules undergo electronic transitions.
The instrument used in ultraviolet-visible spectroscopy is called a UV/vis spectrophotometer . It measures the intensity of light passing through a sample ( I ), and compares it to the intensity of light before it passes through the sample ( I o ). The ratio I / I o is called the transmittance , and is usually expressed as a percentage (%T). The absorbance , A , is based on the transmittance:
A = - l o g (% T )The basic parts of a spectrophotometer are a light source (often an incandescent bulb for the visible wavelengths, or a deuterium arc lamp in the ultraviolet), a holder for the sample, a diffraction grating or monochromator to separate the different wavelengths of light, and a detector. The detector is typically a photodiode or a CCD . Photodiodes are used with monochromators, which filter the light so that only light of a single wavelength reaches the detector. Diffraction gratings are used with CCDs, which collects light of different wavelengths on different pixels. Home
http://bouman.chem.georgetown.edu/S00/handout/spectrometer.htm