Alkenes
Alkenes can form a homologous series of hydrocarbons just like the alkanes. Their general formula is CnH2n .
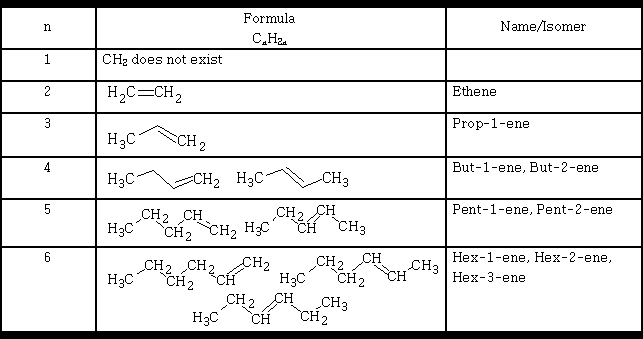
Table 1
In Table 1 we see that isomers are formed, the ones mentioned are referred to as position isomers because of the position of the double bond in the molecule. However, alkenes will exhibit another form of isomerism, that of geometric isomerism . E.g. But-2-ene
![]()
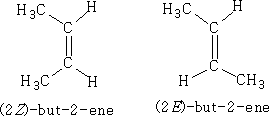
This occurs because there is hindered rotation about the п-bond and the cis(Z) and trans(E) isomers are isolatable. Cis(Z) isomers are on the same side of the п-bond and the trans(E) isomers on opposite sides of the п-bond.
These isomers will have different properties both physical and chemical. Consider fumaric acid and maleic acid

The fumaric acid has a mpt almost twice the maleic acid this is because the fumaric acid can form intermolecular hydrogen bonds with other fumaric acid molecules, the maleic acid only forms intra-molecular bonds.
Intermolecular and Intramolecular bonds:
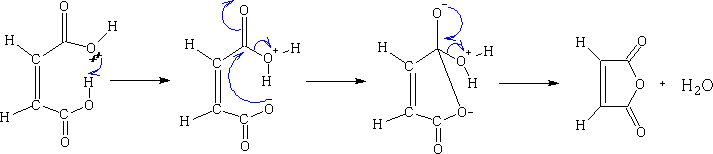
Maleic Anhydride
On dehydration maleic anhydride is formed, fumaric acid forms no such compound. The fumaric acid cannot get into a position to lose a water molecule.
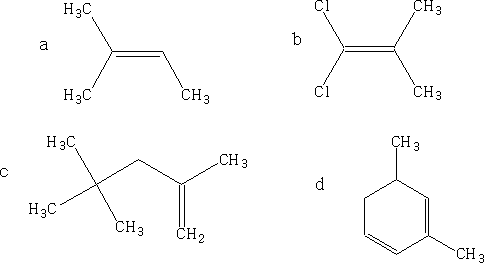
Name the alkenes by the IUPAC system:
Reactions of alkenes
Addition Reactions
1. With H2

This reaction is important in the food industry to make margarines from plant oils.
2. With Halogens: the halogen is dissolved in an organic solvent.
![]()
The bromine colour is discharged.
Mechanism
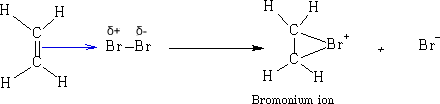
The p-bond becomes more attached to the Brδ+ and a ring structure forms Ďa bromonium ion'. The bromonium ion is then attacked by the bromide ion.
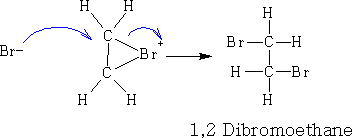
If we now use aqueous Bromine and aqueous sodium chloride instead of bromine in an organic solvent, three products are now formed:
![]()
Each of the products above is formed by a similar mechanism, but because we have additional ions present in the mixture different products are formed.
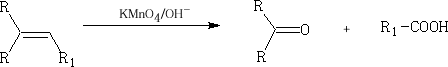
3. With KMnO4 in NaOH(aq)
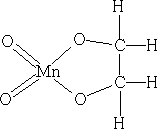
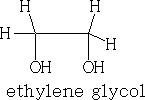
The permanganate (VII) ion forms a cyclic addition product with the alkene, ultimately the C=C bond cleaves to give aldehydes, ketones and carboxylic acids. It can also form the diol, ethylene glycol.
T.W.Bentley Organic Reaction Mechanisms 1973 181-182
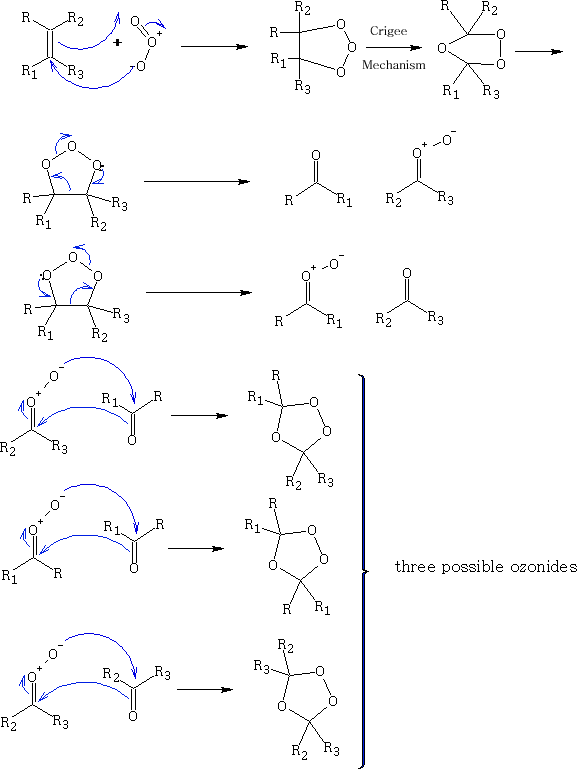
The products so formed are the ketone, aldehyde or acid depending on the R group which are attached to the alkene and the chemical work up.
5. Hydration with concentrated H2SO4
When ethene is bubbled into conc. Sulphuric acid the H2SO4 is added across the C=C following Markownikov's rule and ethyl hydrogensulphate is formed.
![]()
The product ethyl hydrogensulphate when added to water and warmed gives ethanol by hydrolysis.
![]()
6. Industrial production of ethanol by treating ethene with catalyst.
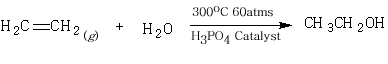
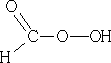
7 Hydroxylation, with performic acid;
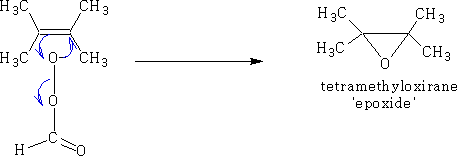
8. Hydroboration, Boron trihydride (as B2H6) then alkaline H2O2
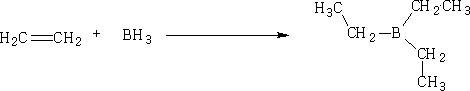
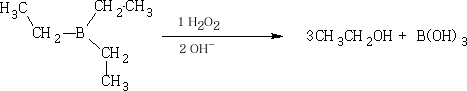
Unsymmetrical alkenes will give different alcohols with greater complexity; the -OH is added Ďanti-Markownikov'.
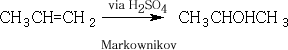
![]()
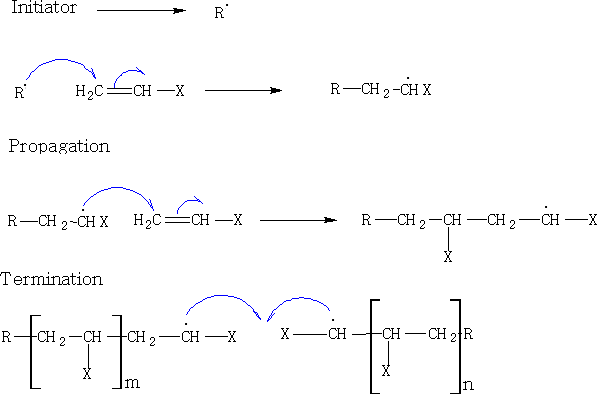
9 Polymerisation
Alkenes undergo polymerisation reactions to form poly(alkenes).
Ethene and propene are polymerised in one of 2 ways
i) Conditions of 200oC and 1500 atms in the presence of a trace of O2
ii) The second is using a Ziegler's catalyst, of which the commonest is aluminium triethyl (AlEt3). The advantage of this method is that it can be conducted at atmospheric pressure, and results in a polymer of high density.
Many other alkenes are polymerised by a free-radical process. The reaction of a mono-substituted alkene occurs as follows:
Alkynes