Alkynes
It takes alkynes to make a world
Nomenclature: CnHn
![]()
ethyne
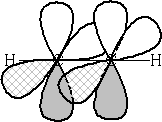
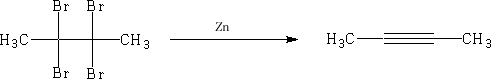
All the alkynes are linear e.g.
It has one s bond and two p bonds orthogonal to one another in the triple bond. See diagram above.
Physical properties:
Mpt's and Bpt's are a little higher than the corresponding alkanes and alkenes.
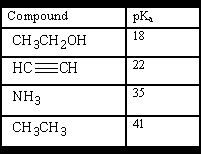
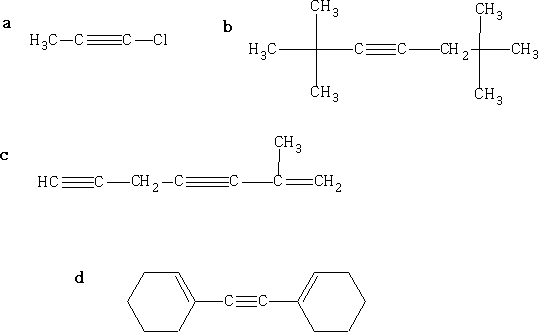
Name each compound by the IUPAC system:
Chemical properties:
1. Additions: These are similar to alkenes; electrophilic or free radical. The intermediate double bond compound can usually be isolated.
![]()
Prop-1-yne will add to:
a) Hydrogen Bromide
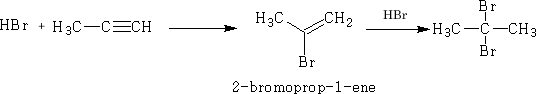
b) Bromine
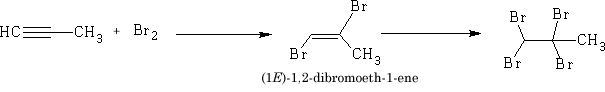
The bromine adds in a similar way to the alkene but because of the steric hindrance the second bromine adds trans to the first bromine.
c) Lindlar catalyst

d) Hydrogen
![]()
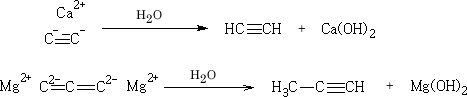
2. 1-Alkynes as acid