The Alkanes, Alkenes and Alkynes
As for butter versus margarine, I trust cows more than chemists.
Joan Gussow (Nutritionist and Educationalist at Columbia University)
Alkanes
Nomenclature : General formula CnH2n+2
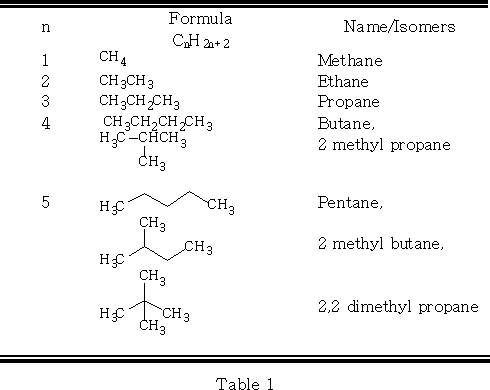
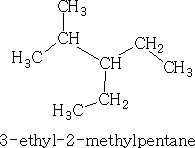
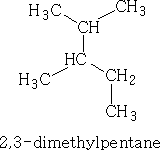
Alkanes are numbered along the longest straight chain. The direction of the numbering is chosen to give the lowest initial prefix number for locating side chains. The side-chain alkyl groups are named in alphabetical order. E.g.
Physical properties:
The straight chain alkanes are gases to C4 , liquids to C17 , and solids thereafter. Branching usually lowers the boiling point; also the melting point decreases if the alkane decreases in symmetry but there will be a rise in the melting point if the alkane increases in symmetry. Cycloalkanes have a higher mpt and bpt than the corresponding n-alkanes.
N.b. The mpt is dependent on the symmetry since symmetrical molecules are packed together tightly and are difficult to separate so the mpt's tend to be higher. The opposite is true for asymmetrical molecules. Hence, n-pentane has mpt = -130oC, 2 methyl butane is = -160oC, and 2,2 dimethyl propane is = -20oC. In general, branching in an alkane will compact a molecule making it more spherical and thus in the liquid state there will be less tangling, branching lowers the bpt. E.g. n-pentane bpt=56oC, 2 methyl butane bpt=28oC and 2,2 dimethyl propane bpt=9oC.
C-C and C-H bonds are strong, resisting homolysis and heterolysis, but homolytic, free-radical reactions are preferred.
Alkane Reactions
1 Chlorination of methane:
Initiation:
![]()
Propagation:
![]()
![]()
Termination:
![]()
Termination reactions are a rare phenomenon since it requires a three body collisions to get this reaction to proceed. Alkanes generally undergo free radical chain reactions such as the ones described above. The termination step is a means by which two chlorine radicals come together, and unless the energy from the bond formation can be distributed to another molecule e.g. those of the container i.e. three body collision, they will possess exactly enough energy to break the bond again.
In the chlorination process the hν radiation must be small i.e. a reaction in diffused daylight, since more Cl. atoms would be formed then were terminated and the chain reaction explodes. With fluorination the chain reaction is impossible to control since F-F is a weak bond and breaks giving heat for even more F-F breakages. Bromine is slow and iodination does not occur at all because there is not enough energy to be gained from the HI formation to initiate.
![]()
Nitration is analogous to chlorination:
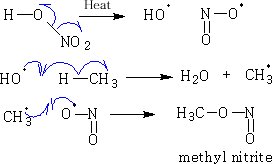
In general, these reactions are not useful since polysubstitution occurs in alkanes more complex than ethane; this is because the free radicals attack different positions giving isomeric products. In most branching molecules e.g. (CH3)3CH, Cl. substitutions take place at the branch point thus the product tends to be (CH3)3CCl, but isomers can still be formed.
2 Thermal Cracking
Initiation:
![]()
Propagation:
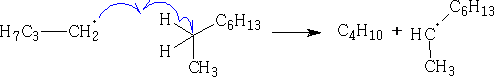
Fragmentation process:
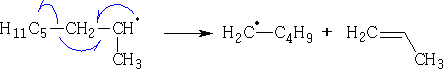
Termination:
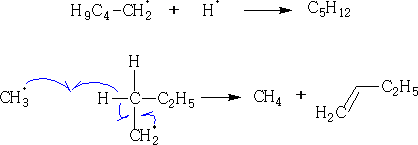
If paraffin is heated up to about 600oC there will be enough energy present to cleave C-C bonds generating free radicals. This process is called thermal cracking. With C18H18 many kinds of free radicals may be formed giving an immense number of possible products especially with long chains. In industry the process is called naphtha-cracking and conditions can be adjusted to give C3 C4 alkenes (starting materials). These reactions are generally used in the electrochemical industry.
3 Catalytic Cracking
Thermal cracking is a free radical process where as a catalytic cracking is an ionic process. There is a similarity between the two processes. The catalysts used in this process are aluminates or silicates. The catalyst enables the formation of carbocations to start the reaction. The usual way is to add small amounts of the compound first; this gives enough carbocation by dissociation.
![]()
a) H - Transfer:
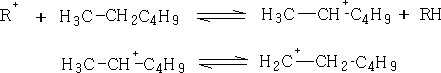
b) Fragmentation:
![]()
c) Rearrangement:
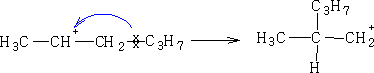
This rearrangement would not occur frequently because the product is less stable then the starting material, this would go to:-
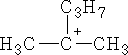
4 Combustion:
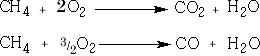
Preparation of alkanes
1. Decarboxylation of acids:
![]()
2. Kolbé electrolysis of carboxylic acids:
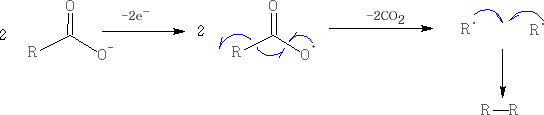
3 Reduction of alkenes or alkynes by hydrogen
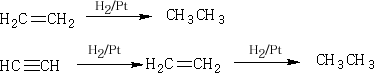
4 Reduction of alkyl Halides
![]()
5 Desulphurisation with Raney Nickel
![]()
Questions:
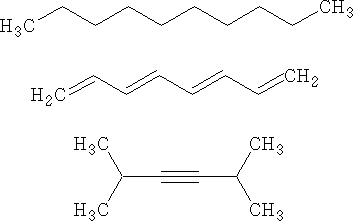
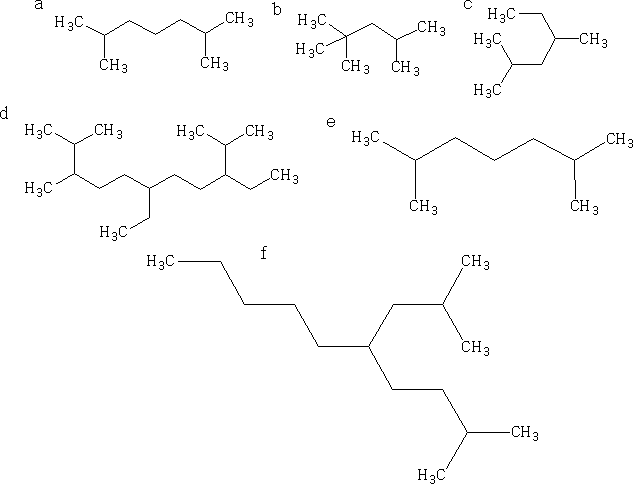
Name each of the structures shown a-f using IUPAC rules.
Alkenes