Primo Levi, The Periodic Table.
Terpenes:
Classification: General Formula – (C5H8)n
n=1 Hemiterpenes
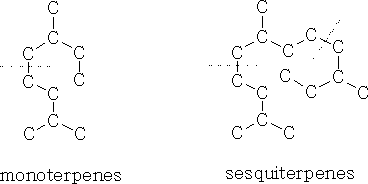
n=2 Monoterpenes
n=3 Sesquiterpenes
n>4 Polyterpenes C40 carotenoids occur in chromoplasts of plants.
Nomenclature:
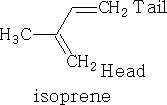
The basic unit for the terpene is the isoprene unit.
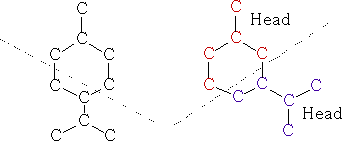
The monocyclicterpenes contain two isoprene units, put head to tail. You can see the individual isoprene units in each structure.
e.g.
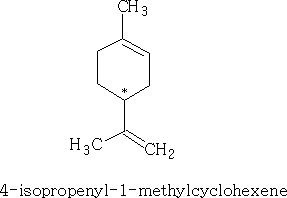
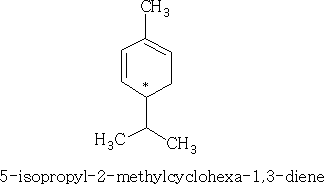
As you can see that each of the substances shown have a chiral centre. If we consider the first structure above we can draw the two optical forms:
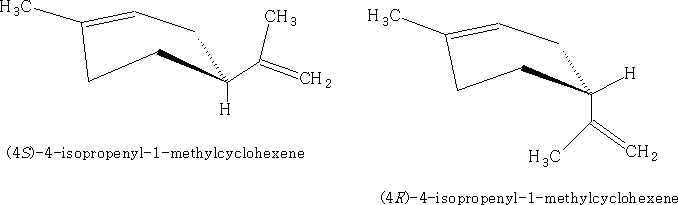
This substance is known as Limonene, the other molecule is called α-phellandrene. Limonene is found in oil of orange, lemon, and bergamot and α-phellandrene in oil of eucalyptus.
Bicyclic sesquiterpenenes are put together in the following way:
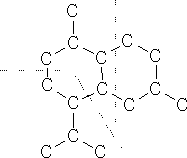
A bicyclic terpene with which you may be familiar occurs in cyclamen.

Alpenveilchen cyclamen
Acyclic terpenes:
These are terpenes which do not have a ring structure; they are represented in a similar way to the cyclic terpenes so that you can see the relationship between the structures.
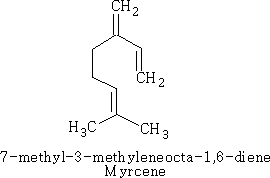

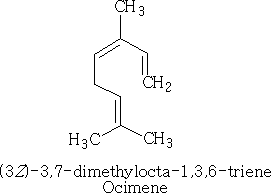
This compound is called myrcene, and is an essential oil which occurs in bay leaves (Laurus nobilis); an isomer of this compound is ocimene, which is the essential oil in Basil.

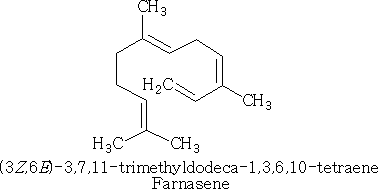
Acyclic sesquiterpenes:
In lemon grass and related plants of the cymbopogon genus, the essential oil citronella can be isolated. The active ingredient in this plant is a-farnesene, which is a sesquiterpene:
Bicyclic terpenes
The bicyclic terpenes have the following structures; the dotted line separates the individual isoprene units.
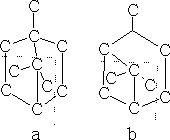
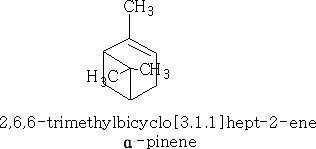
The most important compound is a-pinene (structure b), which is a chief component of turpentine.
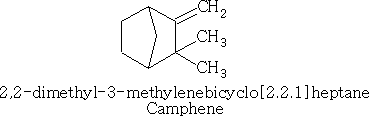
Also camphene (structure a):
Oxygenated terpenes! A great number of oxygen containing compounds exist.
Acyclic: These compounds consist of the basic structure with an addition of an alcohol group or a ketone or aldehyde e.g.
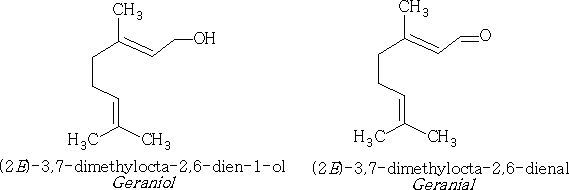
Both geraniol and geranial occur in many plants, i.e. grapes, lemon leaves. Each of these compounds exists with isomers because the CH2OH group and the CHO groups can be cis or trans at carbon 2 e.g.
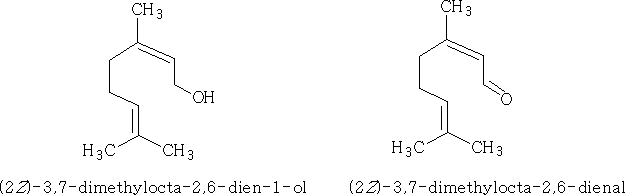
Monocyclic and bicyclic: Similar to above containing the ketone and alcohol groups e.g.
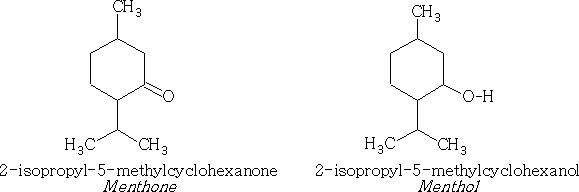
Menthone and menthol are found in peppermint oil.

Camphor and Borneol are ketone and alcohol respectively. Camphor is a well-known bicyclic terpene ketone which has many uses, particularly in medicine and as a plasticiser in nitrocellulose, also used in Vick vapour rub as a decongestant.
we can redraw camphor to show the relative positions of the groups:
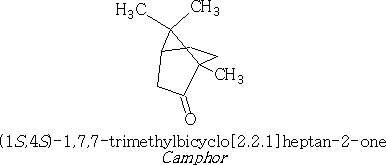
A bicyclic ketone terpene that has gained notoriety over the years is thujone;
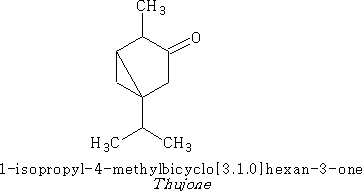
Thujone is one of the toxic ingredients in wormwood which was used to make absinthe, however, it was discovered by analysis that there was not enough of the thujone in absinthe to cause the hallucinations and brain damage suffered by its regular drinkers. It was more likely the excess of the alcohol that did the damage.
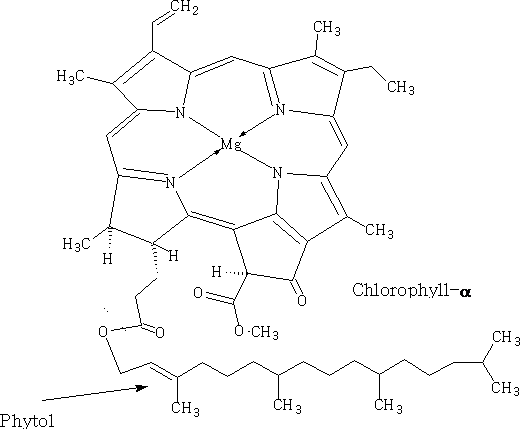
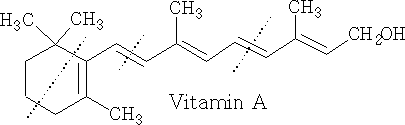
Two important diterpene alcohols are phytol, which occurs as an ester of the side-chain of chlorophyll and vitamin A
 .
.
It should be noted that this structure contains a porphyrin derivative in which the four pyrrole rings are complexed with magnesium ions. The haem structure is related to this, and it is this that makes haemoglobin in the red corpuscles of blood, the central atom in haem is Fe2+.
Vitamin A belongs to the family called the retinoids. Its important part is the retinyl group, which can be found in several forms. In foods of animal origin, the major form of vitamin A is an ester, primarily retinyl palmitate, which is converted to retinol (vitamin A) in the small intestine. The dotted lines indicate the isoprene units.
http://www.rpi.edu/dept/bcbp/molbiochem/MBWeb/mb2/part1/heme.htm http://www.med.unibs.it/~marchesi/heme.html
Terpene rearrangements
Wagner Meerwein rearrangements
These rearrangements are of carbocations:
Winston and D. Trifan, J. Am. Chem. Soc. 71, 2953 (1949); 74, 1147, 1154 (1952)
Rearrangement 1
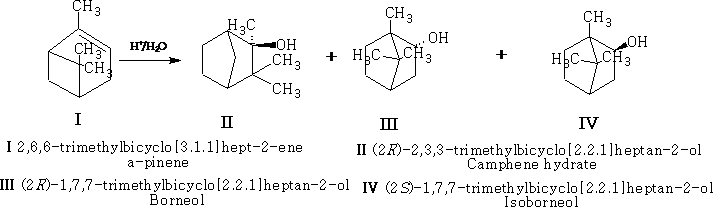
Rearrangement 2
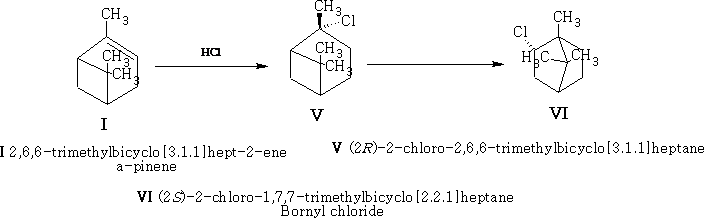
Rearrangement 3
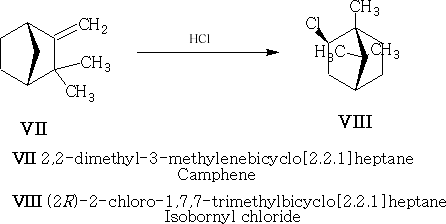
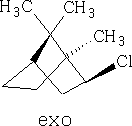
Compound VIII is the exo-compound indicated.
VIII
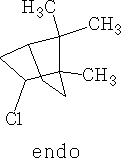
Compound VI is the endo-compound.
VI
The mechanism is as follows for VII to VIII
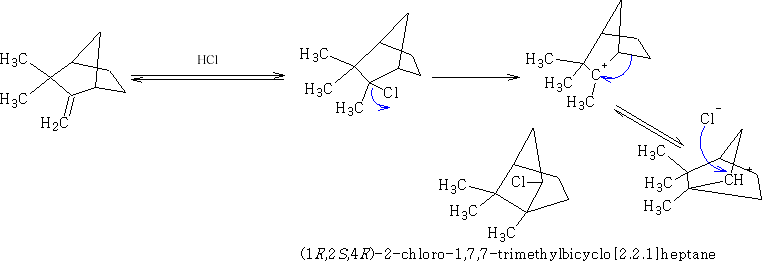
The carbocation is moving about and the chlorine is affected by this ‘windshield wiper effect'.
Cyclisation reactions
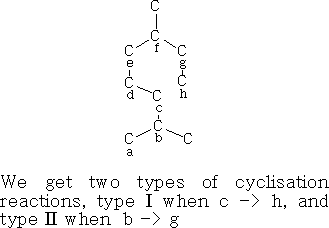
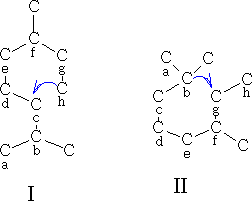
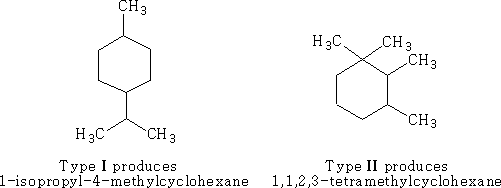
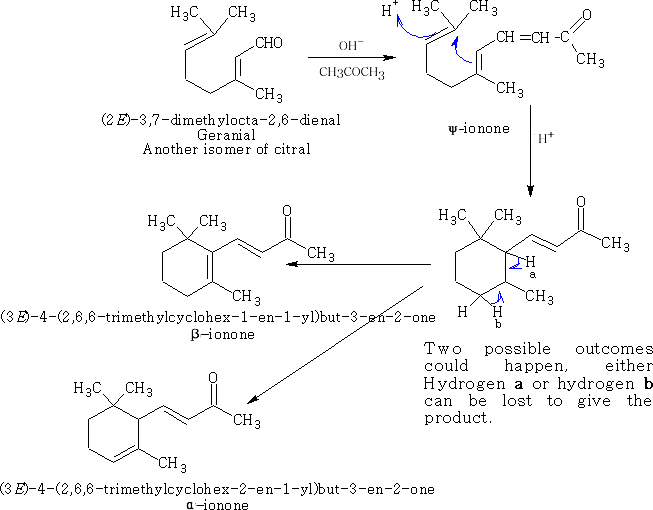
The cyclisation can be done in the following way using oxygen containing terpenes: Type I
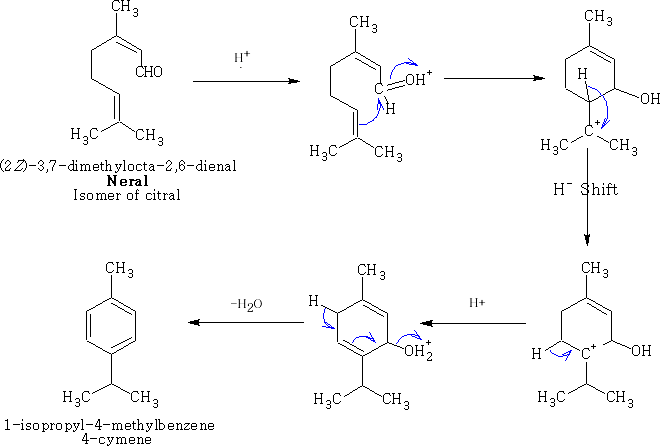
A type II reaction can occur if the oxygen is blocked; the aldehyde is protected with the reaction with acetone: