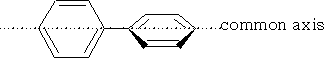
Biphenyls
Biphenyls were first resolved in 1922 and it was not clear at that time as to their structure. The configuration was established by dipole moment and X-ray diffraction data, the assembly proved to be benzene rings which are co-axial.

The molecule cannot be optically active unless the rotation about the common axis can be restricted and the two rings lie in two different planes.
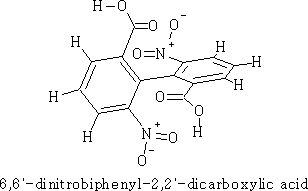
The above molecule has the requirements that make it non-superimposable, and will therefore be optically active. The lack of rotation is due to steric hindrance between the bulky 2, 6 substituents.
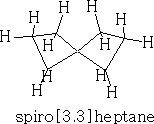
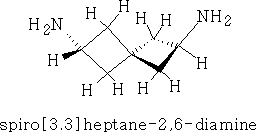
Spiranes
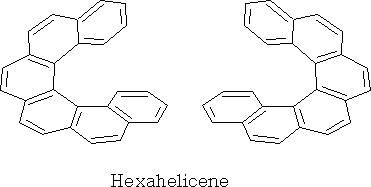
Helicenes
Compounds with an asymmetric atom other than carbon
Many atoms will give asymmetric atoms; nitrogen in an amine has the potential to form optically active compounds. The reason for this is because the nitrogen has a tetrahedral form as follows:
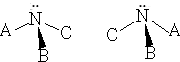
We have three groups at the three corners of a tetrahedron and a lone-pair of electrons at the fourth. The structures shown will be non-superimposable mirror images of one another. Make them to convince yourself!
However, these forms have never been separated into enantiomeric forms. The reason is very simple, the nitrogen molecule flaps too rapidly from one to the other.
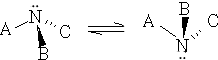
If the flapping is prevented by somehow connecting together A, B and C then optical isomers can exist.
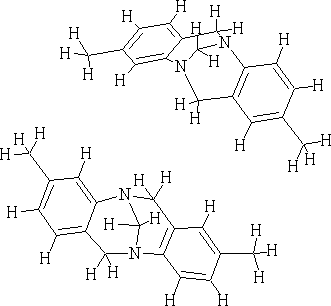
e.g. The following structures are the enantiomers of Tröger's base.
The Structure of Troeger's Base M. A. Spielman J. Am. Chem. Soc. 1935 57(3); 583-585