Chemists are, on the whole, like physicists, only 'less so'. They don't make quite the same wonderful mistakes, and much of what they do is an art, related to cooking, instead of a true science. They have their moments, and their sources of legitimate pride. They don't split atoms, as the physicists do. They join them together, and a very praiseworthy activity that is.
Anthony Standen, Science is a sacred cow (1958).
Electron movements can be described by the use of arrows. A curly arrow represents the movement of two electrons from the tail to the head of the arrow. Thus:
![]()
Reactions involving two-electron shifts are said to involve ionic mechanisms. The electron source is a nucleophile; the electron acceptor an electrophile. Nucleophiles are usually given the abbreviation Nu or Nu- and electrophiles E or E+.
Breaking a bond with both electrons moving to the same end is called Heterolysis, and will involve the formation of electronic charges.
![]()
A one-electron shift is represented by a fish-hook:
Thus: 
Reactions involving one-electron shifts are radical mechanisms . Breaking a bond with one electron moving to each end is called Homolysis . Free radicals are formed which have no charge.
Usually a chemical reaction occurs as a result of an interaction of filled orbitals of one reactant and the vacant orbitals of another, referred to as HOMO-LUMO interactions. Consider two possible scenarios: {Figure 31}
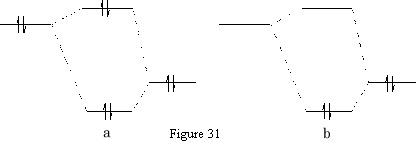
In the reaction between two filled orbitals {Figure 31a} two electrons have been lowered in energy, but this is all cancelled out by the two electrons which have been raised in energy. The interaction between an empty orbital and a filled orbital {Figure 31b} only gives a net lowering of energy since the orbital with the raised energy is unoccupied.
There are many filled and vacant orbitals which can interact; the strongest interaction is between orbitals of similar energies, so the best contender will be between unoccupied molecular orbitals and occupied orbitals which are close in energy.
When several species interact there are two HOMO-LUMO connections possible, the HOMO of the first reactant with the LUMO of the second reactant or, the LUMO of the first reactant with the HOMO of the second reactant. {Figure 32}
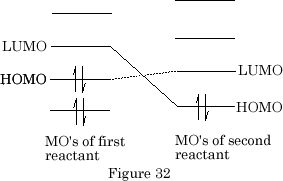
The best interaction is obviously the HOMO of the first reactant with the LUMO of the second reactant as they are separated by the smallest energy difference.
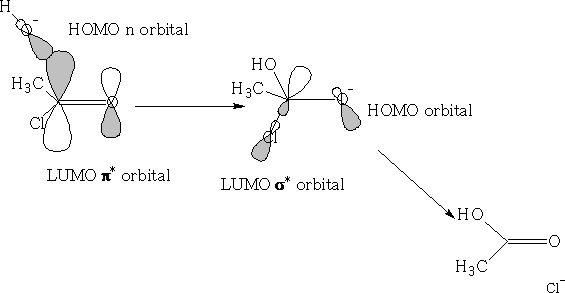
Consider the reaction of a nucleophile with a ketone.
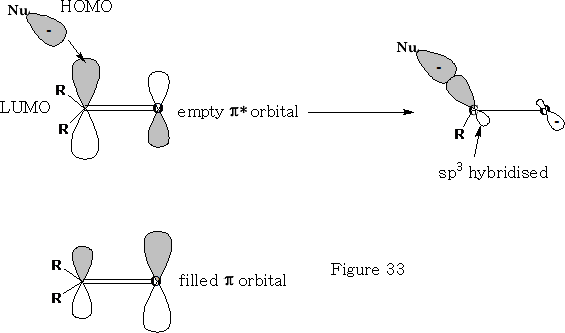
We can see that the HOMO of the Nu comes in at an angle to the ketone, the electrons from the ketone are then pushed onto the oxygen atom, the carbon becomes sp3 hybridised and the oxygen will now pick up an H+ ion, (You will notice that the filled p orbital has a larger orbital over the oxygen atom this is because the oxygen will have the lions share of the electron density, the empty p* orbital is oppositely biased to compensate).
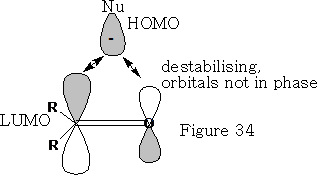
The nucleophile cannot make a direct approach to the ketone as it will get a destabilising effect from the orbital that is on the oxygen {see figure 34} because the orbitals are not in phase.
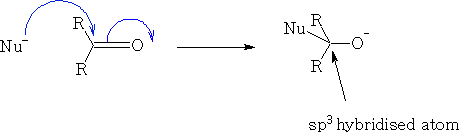
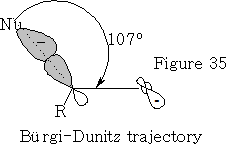
The angle in which the nucleophile approaches is called the Bürgi-Dunitz trajectory, this angle from data is found to be 105±5o see figure 35.
Electrophilic addition to alkenes:
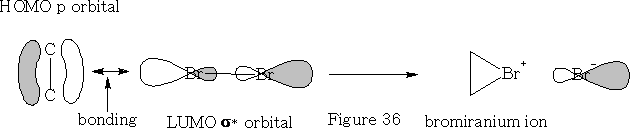
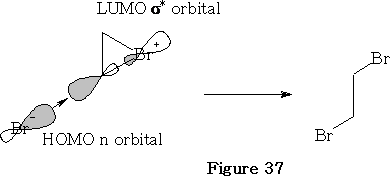
In this reaction the bromine approaches as shown in the figure 36 above. The bromine forms a bromiranium ion with the HOMO of the alkene over-lapping with the LUMO of the Br2 . This bromiranium ion so formed is unstable; the Br - ion now comes in from the back side as the HOMO and 1, 2 dibromoethane results. See figure 37.
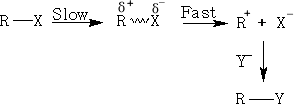
In the Sn1 reaction we have the possibility of the formation of carbocations as transient intermediates. Since stability of a carbocation is attributed to resonance and the formation of a sp2 structure, the more stable the carbocation the more likely it is to go via the Sn1 reaction. The order of stability of carbocations is in the order tertiary>secondary>primary.
Consider: MeBr, EtBr, i-PrBr and t-BuBr
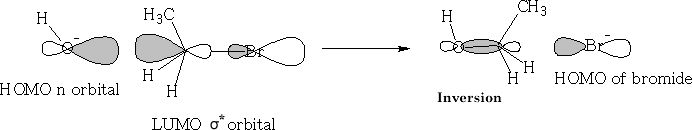
Sn2 (nucleophilic substitution 2nd order)
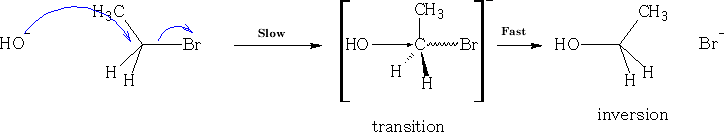
Consider nucleophilic addition reaction. The ketone is reformed after the reaction has completed.
A free radical mechanism involves Initiation, propagation and termination steps
http://www.iupac.org/goldbook/H02851