Nomenclature: These are benzene rings with a hydroxyl group attached.

If we now add substituents to the phenol we get:
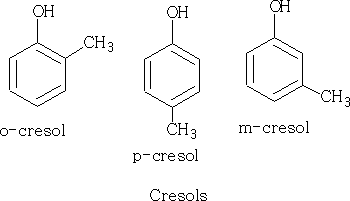
These are 2,-4,-and 6 methyl phenols known as the cresols.
If add two hydroxyl groups we get:
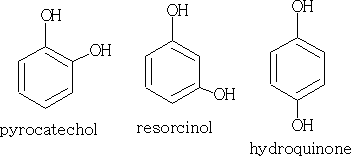
The effect of the ring on the –OH group!
Phenol is a stronger acid than the alcohols because stabilisation of the anion by delocalisation is more important than stabilisation of the parent phenol.
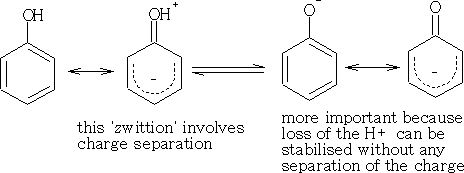
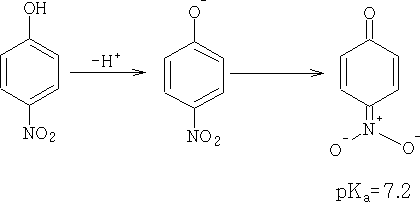
This makes the –OH group more acidic. Electron-withdrawing groups will further stabilise the anion making the phenol a stronger acid.
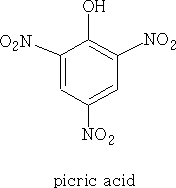
Trinitrophenol (picric acid) has the same pKa as a mineral acid.
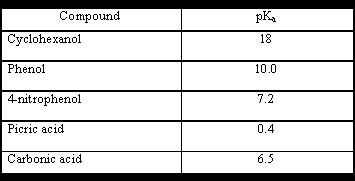
We can see from the table that carbonic acid is a stronger acid than phenol, phenol will not displace CO2 from a solution of a carbonate.
Effect of the oxygen on electrophilic substitution on the aromatic system!
Phenol is a 2,4 directing group: e.g.
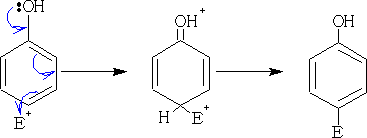
1. Nitration:
Only dilute acid will be required for the nitration of phenol, nitric acid contains a small amount of nitrous acid which because of the activation of the ring will be more than enough to nitrate the phenol. Two products are formed, 2-nitrophenol and 4-nitrophenol.
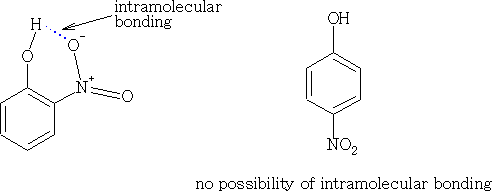
The effect of the Intramolecular bonding makes these two compounds easy to separate, steam distillation of the reaction liquid will give the 2-nitrophenol first. Why? (Mpt 2-nitrophenol 45oC; mpt 4-nitrophenol 114oC).
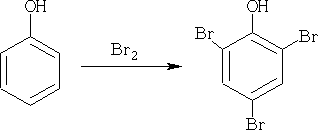
2. Halogenation:
Halogenation is very fast, and tends to produce the 2, 4, 6 product.
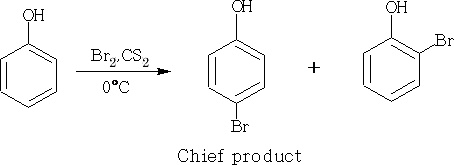
This reaction can be slowed down if CS2 is used as solvent, and the monohalogen product can be made.
3. Friedel Crafts: This has been covered in an earlier section, the phenols however, require less active sources of carbocation, the catalyst AlCl3 tends to complex with the phenol so it is not used, and in fact is not required in this instance.
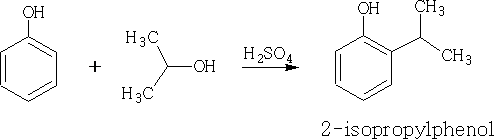
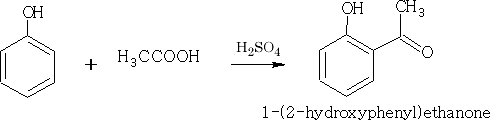
The yields for these compounds in each case are poor.
4. Nitrosation
Nitrous acid converts the phenol into nitrosophenols.
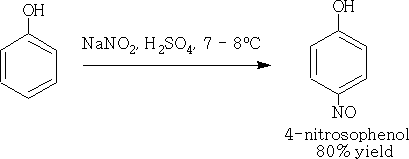
Phenols are one of the few compounds which are attack by the weak electrophilic nitrosonium ion (NO+).
If the reaction is allowed to proceed at a higher temperature a further reaction happens coupling the nitrosophenol with phenol.
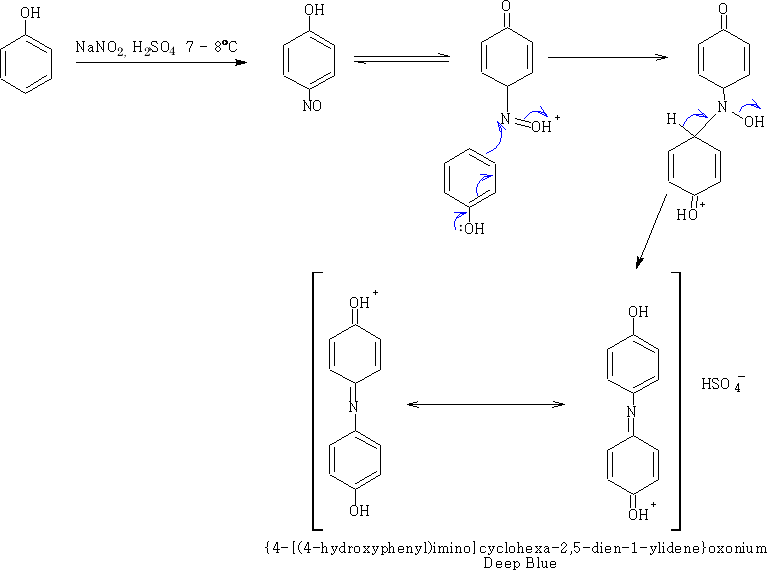
This is ‘the Liebermann nitroso reaction', the uncharged ‘indophenol' is red; delocalisation is less important and the compound is less deeply coloured. But in alkali the compound becomes deep blue again. This can be used as a test for phenols.
5. Diazo-coupling: With diazonium chloride we get the diazene.
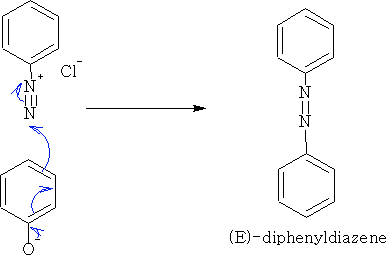
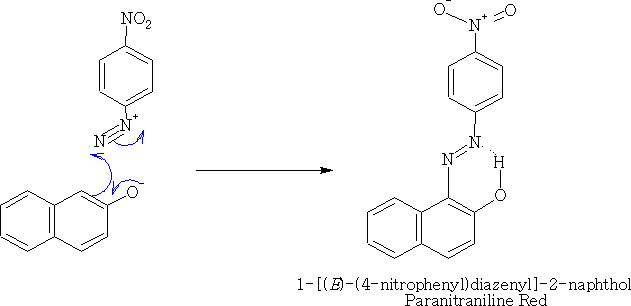
Reations with more complicated phenols gives the class of compound called the azo dyes e.g.
6. Reactions with carbonyl and related compounds
i) Aldehydes and ketones react with either acidic or basic catalysts e.g Hydromethylation
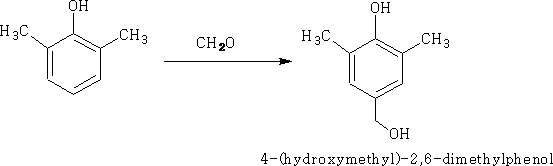
Under more vigorous conditions a further reaction will take place,
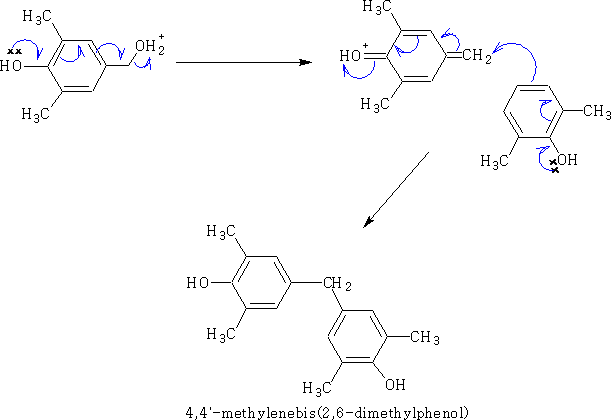
This process is the important reaction which will form phenol-formaldehyde resins. (e.g. Bakelite)

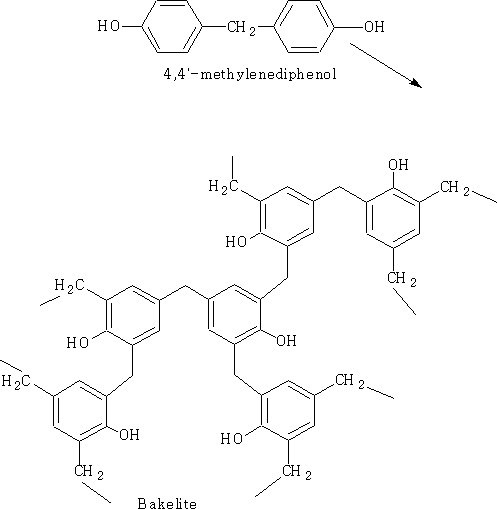
The polymerisation of Bakelite resin is usually carried out to a relatively low stage of completion and then the low melting pre-polymer (resole) is put into a mould and heated to give the final product.
ii) With CH2O/HCl:
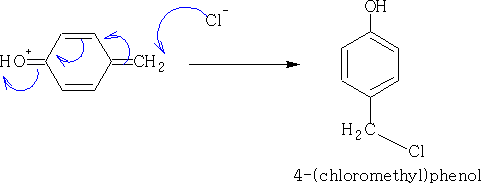
iii With CH2O and a secondary amine hydrochloride
Initially N -methyl- N -methylenemethanaminium chloride is made:
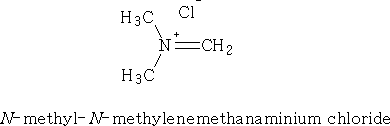
This then reacts with the phenol to give:
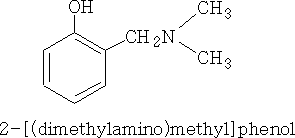
This is called the Mannich reaction.
iv With CO2 and phenoxide. Kolbé-Schmitt reaction:
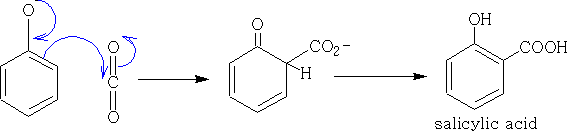
The mechanism shown is one way that this compound could be formed, a second is called the Morasse modification of the Kolbé-Schmitt reaction and uses anhydrous potassium carbonate in an atmosphere of CO2 at a pressure of 1200psi and 175oC to give the product.
Preparation of phenols
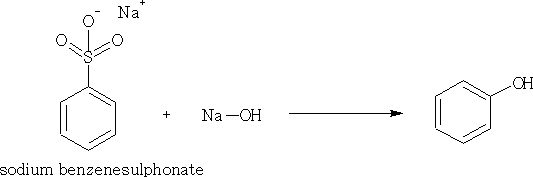
1. Fusion of sodium benzene sulphonate with NaOH
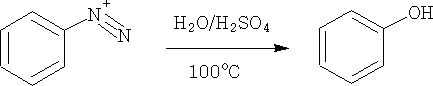
2. From diazonium salts:
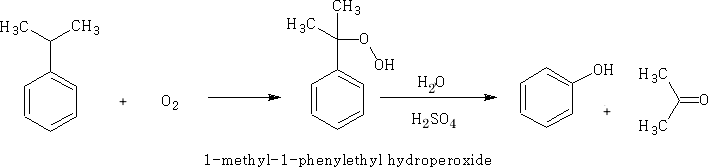
3 From Cumene and oxygen.