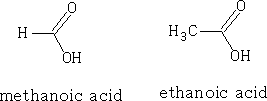
When a bond is strongly polarised (i.e. it has two different atoms joined together) it has a charge separation and is said to have a dipole moment. This permanent dipole can have an effect on adjacent bonds; the effect of this permanent dipole induced by one bond in another is called the inductive effect . This effect falls off with distance. We can see the consequence of the inductive effect in the following example: -
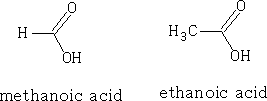
If we compare the acidities of the two acids we find that pK formic =+3.74 and pK ethanoic =+4.76. N.b. the smaller the pka the stronger the acid.
How does this come about? To find out we need to look at the properties of the different groups in their ability to pull or push electrons.
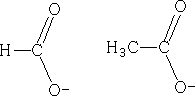
Lets look at the ions that we have above; the structures could equally be:
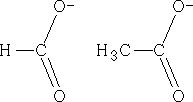
The charge could be distributed over either oxygen, in fact, the charge is:

This is known as delocalisation. This results in unexpected bond lengths both structures above imply that there should be two carbon oxygen bonds of different lengths, single bonds are longer than double bonds, but delocalisation means that both of these bonds are of equal length. Also, double bonds have a greater density of electrons than single bonds, so they should repel the single bonds more than they repel each other, resulting in different bond angles within molecules such as these. However, due to delocalisation, the bond angles are the same. Why are the pKa values different?
This is a case of Hyperconjugation, (see later) the methyl group has a tendency to push electrons into the carboxylic acid group, making this COOH group more electron rich, the electron has less tendency to leave and the acid is weaker.
The hydrogen ions H+ are placed as shown in figure 16, and the ve charge is equally spaced over the carboxylic group. The ability for the molecule to lose the H + is dependant on the way that the substituent groups push or pull electrons. H as a substituent group neither pushes nor pulls electrons; however, alkyl groups have a tendency to push electrons (+I effect). This has the effect of making the carboxylic acid group more electron rich and so the H+ will have less tendency to leave and produce H3O+ ions (hydroxonium ions).
Halogen atoms are good at pulling electrons towards them, so they have a good I effect. Consider the following that of chloroethanoic acid:
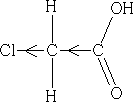
What do we expect for the acidity of this acid? pKaCEA =+2.86. It is more acidic than ethanoic acid! How do we explain this??
The chlorine atom draws electrons towards itself thereby making the carboxylic acid group less electron rich, the H+ will have less attraction to the acid group and so can leave more easily, the acid is more acidic. How do we expect the acidity to change if we add another Cl atom to give dichloroethanoic acid?
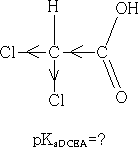
Consider:
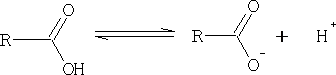
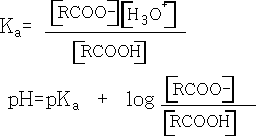
We can see from this equation that the bigger the pKa value the weaker is the acid.
In aromatic acids, the mesomeric effect also has a roll to play in the acidity of the acid, and along with the inductive effect will influence the acidity of the compound.
The mesomeric effect is the electron withdrawing or electron releasing effect of substituent groups on a compound. The effect can be positive (+M ) when the group is electron releasing or negative (-M) for a electron withdrawing group.
Benzoic acid pK a = 4.2
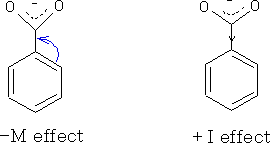
Both the mesomeric and inductive effect plays a part in the acidity. In benzoic acid these two effects virtually cancel one another out. However, in substituted benzoic acids the mesomeric effect can be seen properly.
e.g.
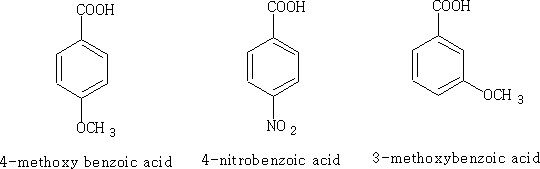
Let's consider each of the substance above: i)
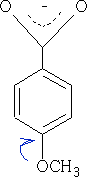
Electrons are pushed into the ring from the - OCH3 group hence the acid has a surplus of electrons in the ring and the acid becomes less acidic pKa = +4.5, the +M effect is greater than the I effect of the oxygen in the CH3O- group.
ii)
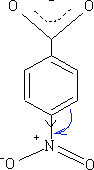
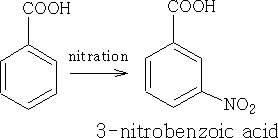
The NO2 group has both an M and I effect on the benzoic acid, the pKa for this molecule is +3.4 and obviously more acidic.
iii)
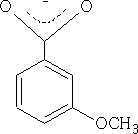
Because the OCH3 is attached to position 3 it does not have access to the mesomeric effect only the inductive effect plays a role here. The acid is therefore more acidic pKa = +4.0 than benzoic acid.
So far we have seen the delocalisation of electrons in the π system; however, in some cases we get a delocalisation of s electrons into the π system this is referred to as Hyperconjugation. We have seen that methyl groups push electrons into carboxyl groups this is Hyperconjugation. So in effect it is an interaction of the s bonds with the π network of the carboxylic acid. How does this affect the benzene ring? Consider: Methyl benzene (toluene)
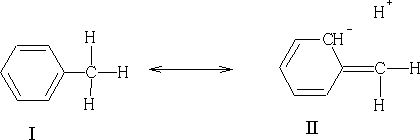
In this example we see that the H+ is not bonded to the carbon, this resonance is known as no-bond resonance . Hydrogen does not leave because II does not exist at all; it is only a form which contributes to the actual structure of the molecule. The effect of II is that the electrons of the C-H bond are closer to the carbon atom. This has an effect on the position in which electrophilic attack can occur on the benzene ring because the methyl group will activate certain positions relative to the substituent group. In methyl benzene the positions which are activated will be the 2,4 and 6 position. If you look at structure II above you will see that position 2 has got a negative charge, this of course, does not exist (it is more likely to be a d- charge), but it will make this position more electron rich. By conjugation this can be transferred to positions 4 and 6.
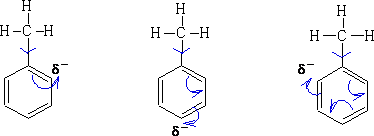
So on reaction Wheland intermediates will be produced similar to those seen in benzene. e.g. Nitration of methyl benzene:
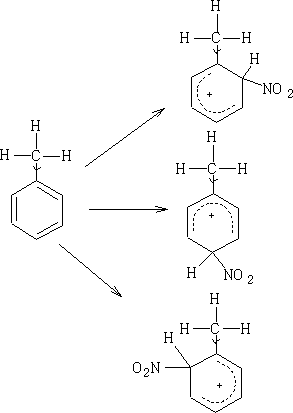
The NO2+ ion will attack at the most negative centres. Hence we will get only 1-methyl 2 and 4-nitro benzenes (1-methyl-6-nitro benzene is the same as 1-methyl-2-nitro benzene) and 1-methyl-3-nitro benzene is not formed in any significant quantity.
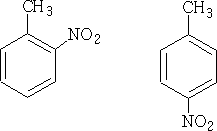
By and large if a substituent pushes electrons into the ring it will direct the reaction to 2,4 products, and will generally react faster then benzene itself.
Consider: Chlorobenzene.
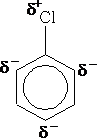
The chlorine atom on the ring has the effect of withdrawing electrons from the ring; hence it will slow the reactions that the ring undergoes. It is said to deactivate the ring. The presence of the Cl atom however, will still direct the ring to react at the 2,4 positions. e.g.
Consider, Phenol and Phenylamine:
These substances react a lot faster than benzene; this is because of the lone pairs of electrons on the oxygen and nitrogen atoms. These are fed into the π system and the ring is activated. The positions activated are again 2, 4.
We have so far only seen the effect of the activating influence of the substituent group.
Consider Benzoic acid:
This group is now working in the opposite direction to the other groups so far looked at, what effect has it on the direction of reaction? The 2, 4, 6 position all now get a positive charge associated with them. The E+ cannot now attack at these positions, hence the only positions which have a more negative charge are the 3, 5 positions.
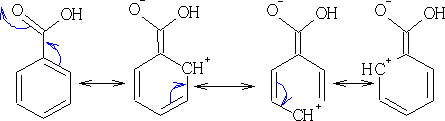
This group has a tendency to deactivate the ring and direct reaction towards the 3, 5 positions.