Heterocyclic Chemistry
This is a huge and varied topic, many types of heterocyclic compounds exist, the main elements or more specifically 'heteroatoms' that are incorporated into the molecules are nitrogen, oxygen and sulphur. Many important heterocyclic compounds include dyestuffs, flavenols, alkaloids and anthrocyanins.
The following collection of molecules gives you an idea of the vast and extensive scope of this field.
I have separated them into families of molecules so that I can look at these individually.
Furans
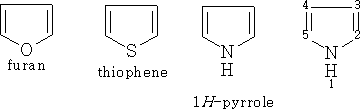
The properties of these compounds have a resemblance to benzene chemistry although the rings will decompose much more easily. In the case of thiophene its properties are very close to that of benzene and it is extremely difficult to separate from a mixture.
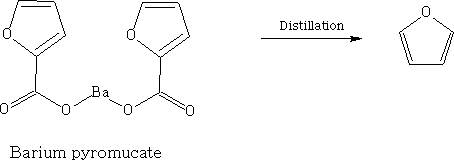
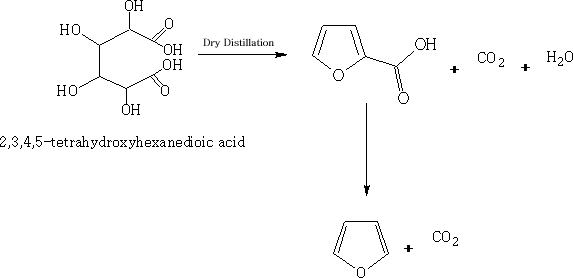
Furan occurs in wood tar and in pine-wood distillate and can be made by the distillation of Barium pyromucate.
It may be prepared by the dry distillation of mucic acid, and heating the product furoic acid at its boiling point.
Properties
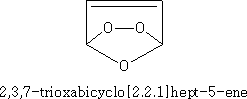
This substance will polymerise.
Furan can be reduced by Raney Ni and hydrogen gas to tetrahydrofuran (THF) in about 90% yield.
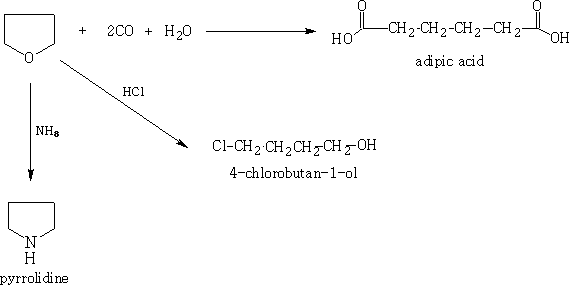
THF is a very useful substance as it is used as a solvent and can be reacted with CO and water to form adipic acid.
It forms pyrrolidene with ammonia and 4-chlorobutan-1-ol with HCl.
Reactions
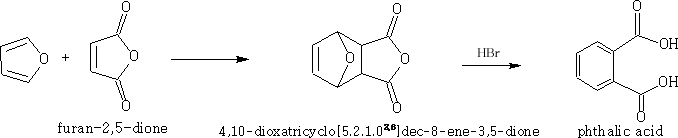
a) Furan undergoes a Diels-Alder reaction forming after reaction with hydrobromic acid, phthalic acid:
b) Furan undergoes the Gattermann formylation reaction1 to form furfural.
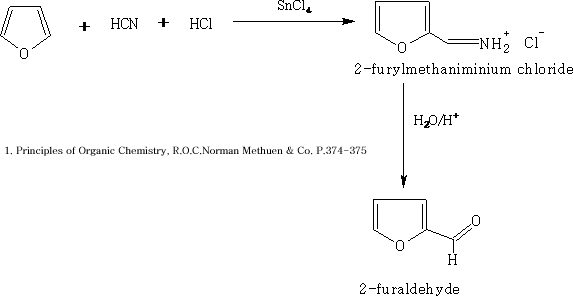
Since AlCl3 reacts with the furan, SnCl4 is used as the Lewis acid.
c) Furan will form organometallic compounds with n-butyl lithium which can be used as intermediates in the synthesis of furan derivatives. E.g.
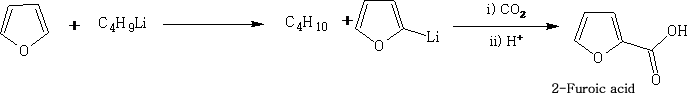
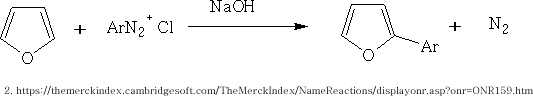
Just like benzene the furan will react with the diazonium salt in the presence of alkali to form an adduct of homolytic arylation.
Thiophene
Properties
Thiophene has a b.pt of 84oC and a m.pt of -38oC, it is a colourless liquid which has no smell reminiscent of the thioethers.
Preparation
Thiophene can be prepared by passing a mixture of ethyne and hydrogen sulphide over alumina at 400oC
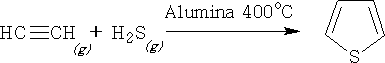
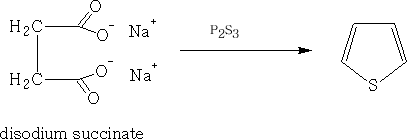
It also can be prepared by heating sodium succinate with phosphorus trisulphide.
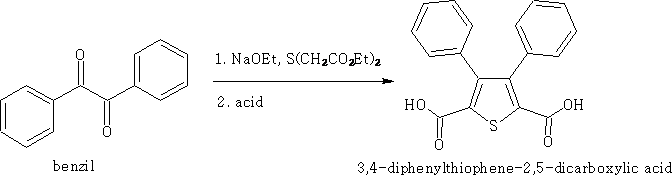
The Hinsberg's procedure3

An α-dicarbonyl compond is condensed with a thioether in which the sulphur atom is next to methylene groups which have been activated.
e.g.
Heterocycles, 2009, vol 78,# 10 p 2489-2507
The Fiesselmann Thiophene Synthesis4
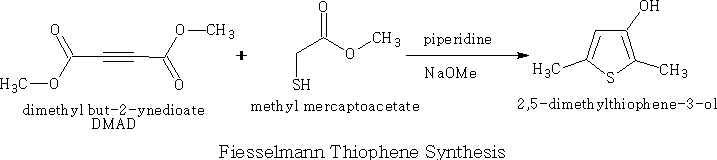
Methyl mercaptoacetate is treated with DMAD in piperidine followed by sodium methoxide, the substituted thiophene is produced in good yield.
Tetrohedron Letters,2008, vol 49 #15, p. 2425-2428
Reactions
Acylation by acetyl chloride causes the thiophene to be polymerised, so instead of using AlCl3 the best lewis acid is SnCl4 as it is much weaker than the aluminium chloride.
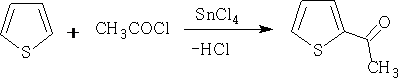
Chlorination:
Thiophen can be chlorinated at low temperatures, and at -30oC will give the 2-chloro derivative and the 2,5 derivative.
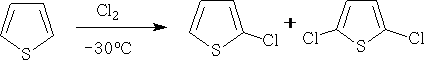
Nitration:
Thiophene can be nitrated easily using a mixture of nitric acid and ethanoic acid.
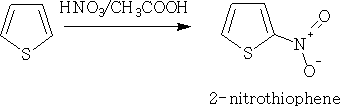
Sulphonation:
Thiophene can be sulphonated using 1-protopyridinium suphonate to give a 90% yield, 95% sulphuric acid can be used but the yields is not so great.
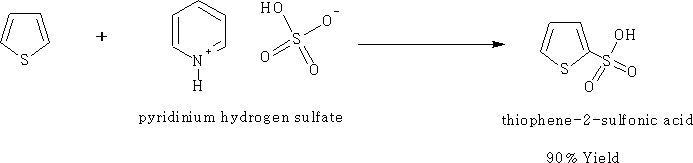
Pyrrole
Properties
Pyrrole has a m.pt of -23oC and a b.pt of 129-131oC.
Preparation:
Barton-Zard Pyrrole synthesis5
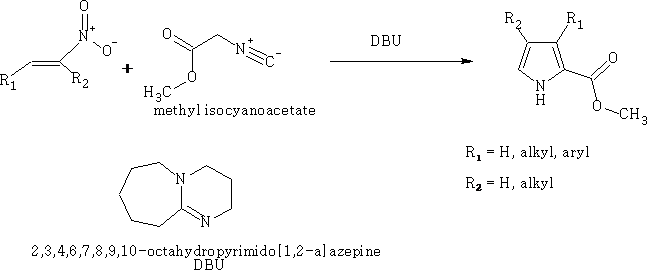
This reaction involves the reaction of the α-isocyanide acetate with a nitroethene compound, the substituted pyrrole is formed as shown, the mechanism is as follows:
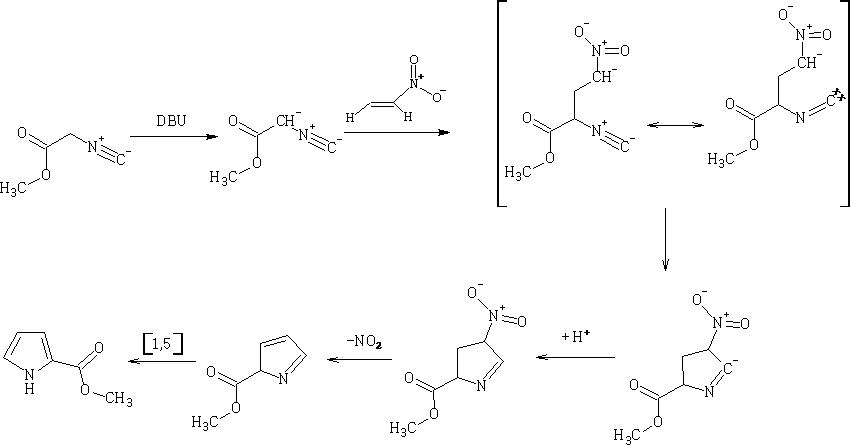
Try to work out the movement of electrons in the mechanism??
Piloty Pyrrole synthesis6
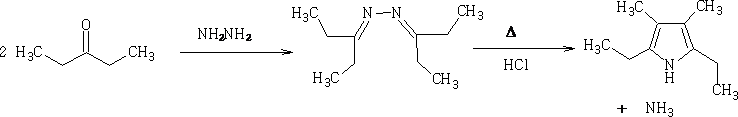
Mechanism:
Pyrrole Synthesis by a combination of ring-closing metathesis an in-situ oxidative aromatisation7:
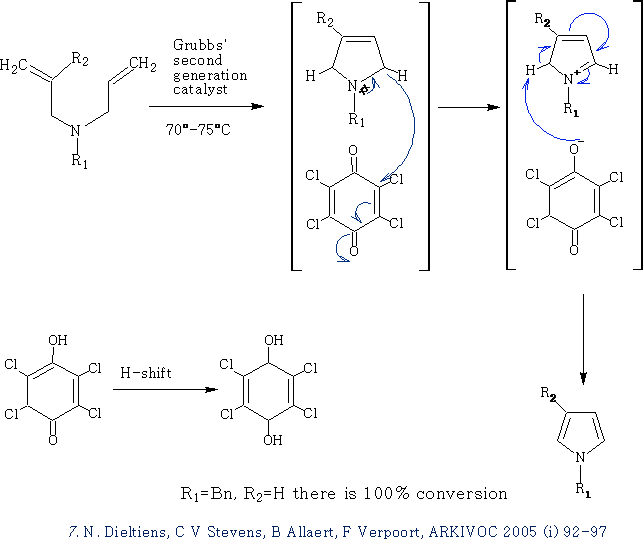
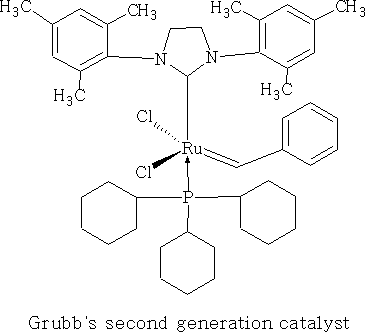
This reaction uses the Grubbs' second generation catalyst,
The tetrachloro-1,4-benzoquinone is a strong H acceptor and it speeds up the aromatization reaction.
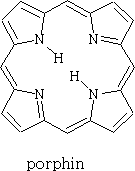
Porphyrins:
Porphin is the parent of the porphyrin compounds which include Haemin (haemaglobin) and chlorophyll.
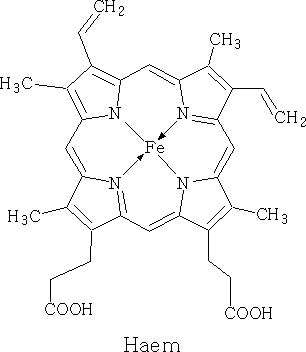
Chlorophyll has different forms, it occurs as chlorophyll-a, b, c and d. It is the green pigment in plants, algae and cyanobacteria. Chlorophyll is responsible for photosynthesis which allows the water and carbon dioxide to be turned into sugars using light.
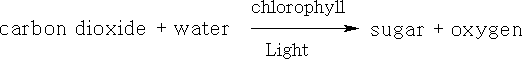
In its action one molecule of chlorophyll absorbs one photon and releases one electron.
Chlorophyll has the following forms:
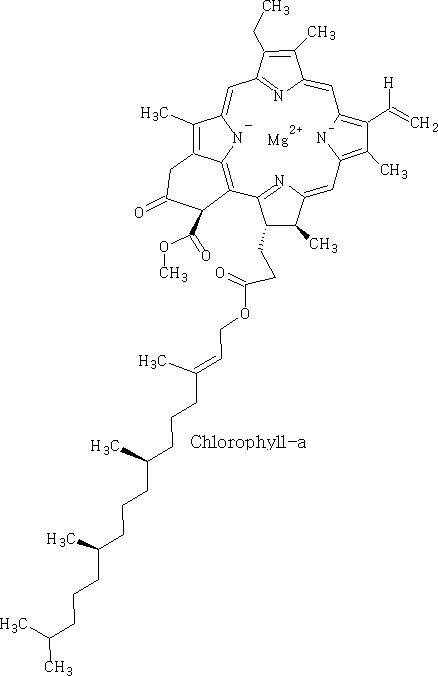
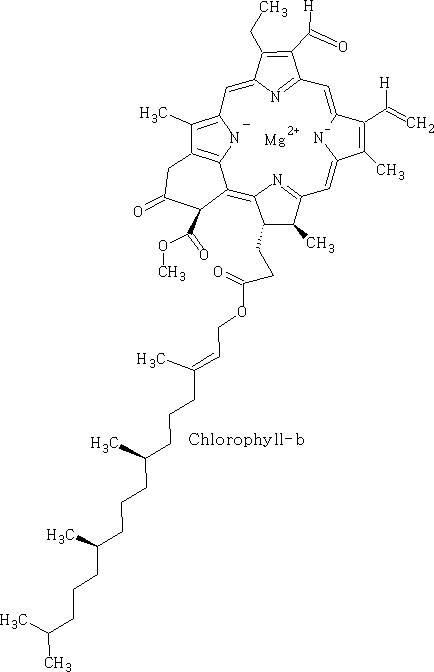
These forms above are the main compounds in plants, chlorophyll-c and d occur mostly in algae and cyanobacteria.