
Numbering:

The existence of a circle of electrons gives rise to a ring current just like benzene. Naphthalene is more reactive than benzene.
The resonance energy for naphthalene is 250 KJmole-1
Resonance structures are:
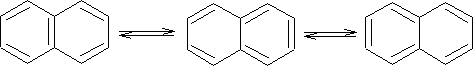
X-ray analysis of naphthalene shows that it is not like benzene in that its bonds are not all the same length. i.e. C1-C2 bond is 136.5pm and the C2-C3 is 140.4pm, we see that C1-C2 is a double bond in two structures and C2-C3 is single in two structures.
Electrophilic attack on the naphthalene ring;
E+ can attack at both the 1 or 2 position on the ring, but which is prefered?
Consider:
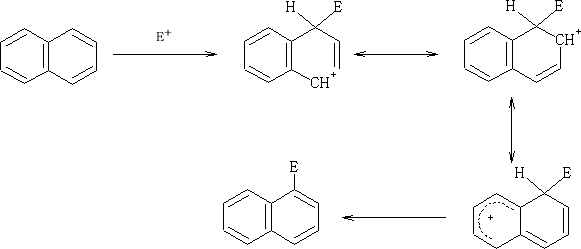
The positive charge that is formed when the E+ is added to the 1 position is distributed over two carbon atoms and does not affect the resonance in the other ring. Contrast the addition of the E+ at the 2 position.
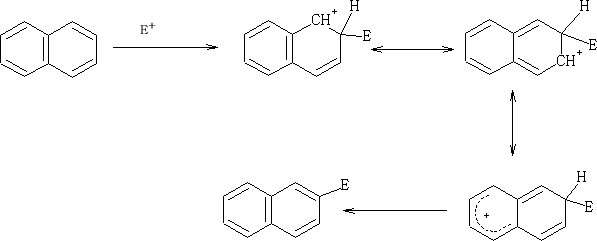
Here the second rings resonace is disturbed by the formation of the positive charge which is distributed over the two carbon atoms.
So in this position the one which have the greater probability of being attacked is at the 1 position.
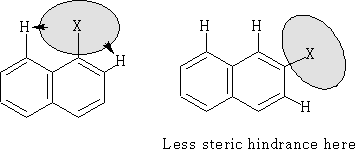
There is one other factor that will also effect the position of the substitution; that is the size of the group being added. In the case of large groups the ß position is favoured. This is a case of steric hindrance with the hydrogen's on neighbouring carbon atoms.
Nitration of Naphthalene
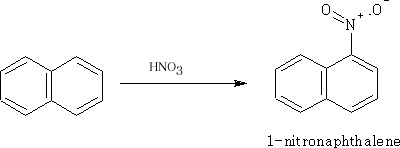
The 1-position is the major product.
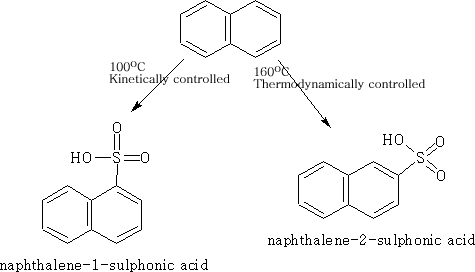
Sulphonation of Naphthalene
Because of the large size of the suphonate there is a steric factor which is now operating and by changing the conditions we can change the product depending on whether we use kinetic or thermodynamic conditions.