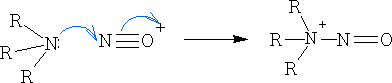
Amines
The amine bond is polarised as shown:
![]()
The lower the electronegativity of the nitrogen compared to the oxygen means that carbon is less susceptible to nucleophilic attack and that the nitrogen lone-pair is more available to react as a nucleophile.
Nomenclature:
RNH2 primary amines
R2NH secoondary amines
R3N tertiary amines
R4N+ quarternary amines
Physical properties
N-H.......N hydrogen bonding is weaker then O-H.......O bonding; so 1o and 2o amines have higher melting points and boiling points than hydrocarbons of the same molecular weight.
Chemical properties
Acidity of amines:
Amines ionise with proton loss less easily than alcohols,i.e. they are weaker acids. Very strong bases such as CH3- will remove a proton. (known as the Zerewitinoff determination of active hydrogen; Methyl Grignard reagent is added to the amine and the amount of methane produced can be determined quantitatively*)
* Determination of methane by catalytic oxidation, Ind. Eng. Chem. Anal. Ed. , 1935 , 7 (1), pp 9–11 DOI: 10.1021/ac50093a006
![]()
Alkali metals dissolve in amines, but require heat to initiate a reaction with them.
![]()
pKa(amine)=35 this a weak acid.
Basicity of amines:
Basicity is measured as pKb, or more usually now as pKa of the conjugate acid, i.e. pKBH+
Amines are moderately strong bases, e.g. EtNH2 , pKBH+=10.7
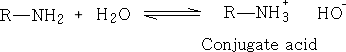
an acid is:
![]()
where A- is the conjugate base.
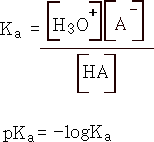
Reactions of amines:
Nitrous acid
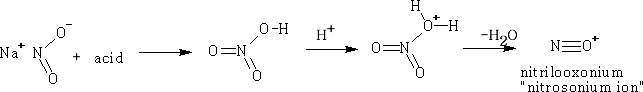
Consider the reactions of each class of amine with the 'nitrosonium ion',
a) R3N